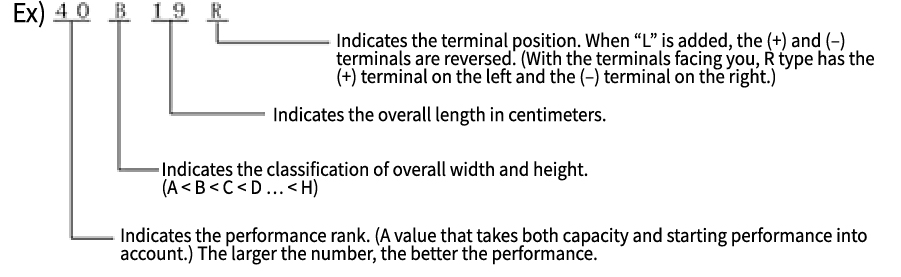
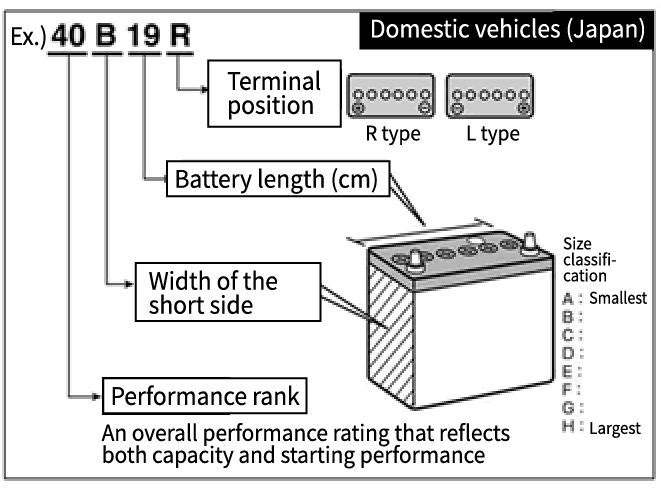
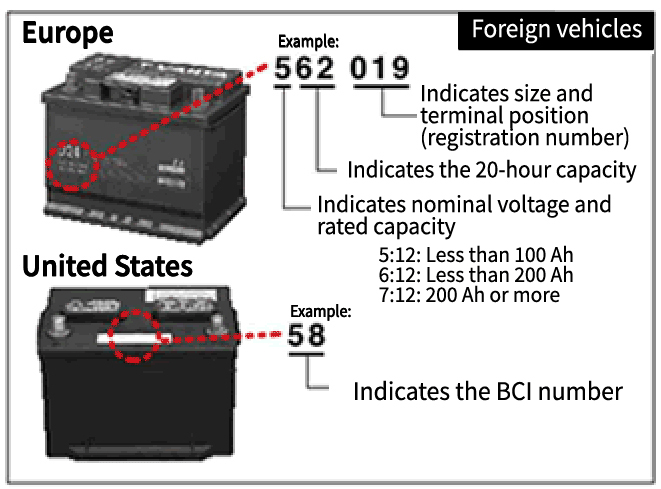
Basic Knowledge of Automotive Batteries
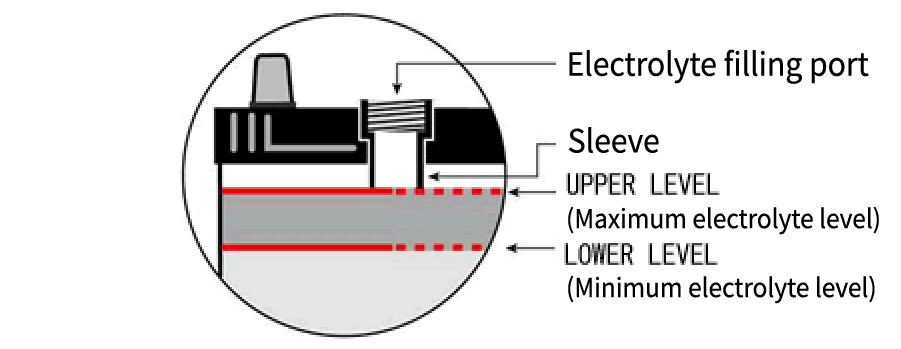
Electrolyte Level Check
Check the electrolyte level from the side of the battery.
If the level has dropped to half or below between the “UPPER LEVEL” (maximum level line) and “LOWER LEVEL” (minimum level line), immediately refill with purified water (commercially available battery refill water) up to the “UPPER LEVEL.”
After refilling, make sure to tighten the vent caps securely.
※ Electrolyte level inspection is legally required as part of daily maintenance under the Road Transport Vehicles Act . Please check at least once a month.
Specific Gravity Check
If the specific gravity is 1.240 (at 20°C) or below, recharge the battery.
Connection Check
If terminals are loose or corroded, resistance increases, which may cause hard starting or terminal damage due to sparking.
Ensure terminals are properly tightened.
If corrosion is found on the terminals, clean them thoroughly.
Appearance Check
Check the battery body for cracks, breaks, chips, electrolyte leakage, and make sure no dirt or debris is clogging the vent holes of the caps.
Dirt or moisture on the top surface can cause leakage, so wipe it clean with a damp cloth.
Do NOT wipe with a dry cloth, as static electricity may cause the battery to rupture.
Installation Check
Secure the battery firmly using mounting brackets to prevent vibration.
To prevent short-circuiting with tools, follow the steps below.
Incorrect order may result in battery explosion or injury.
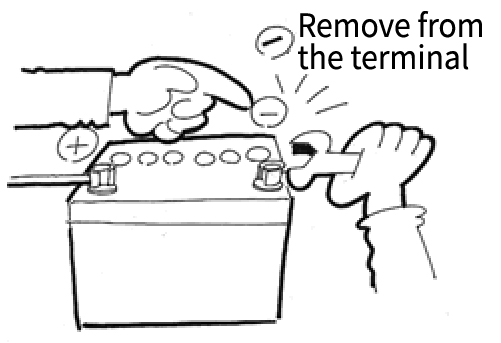
When Removing
Disconnect the negative (-) terminal
→Disconnect the positive (+) terminal
→Remove the mounting brackets
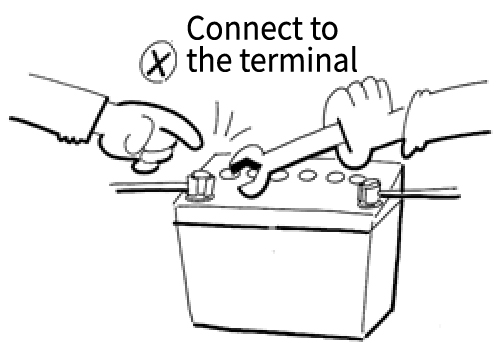
When Installing
Install the mounting brackets
→Connect the positive (+) terminal
→Connect the negative (-) terminal
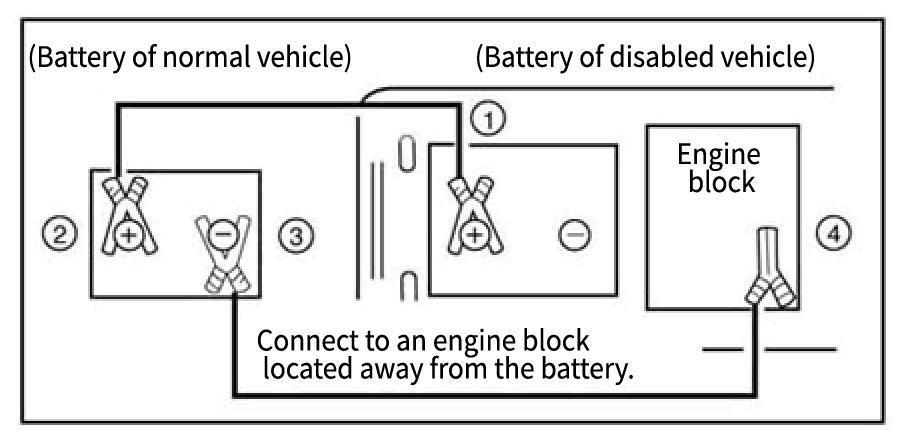
- Correct Connection Procedure:
Follow steps (1) → (4) in the specified order. - Correct Removal Procedure:
Remove in the same order as (1) → (4).
If the supporting vehicle’s battery is smaller in capacity than the stalled vehicle’s battery, the internal components of the supporting battery may be damaged.
Battery capacity (size): Supporting vehicle’s battery ≥ Stalled vehicle’s battery
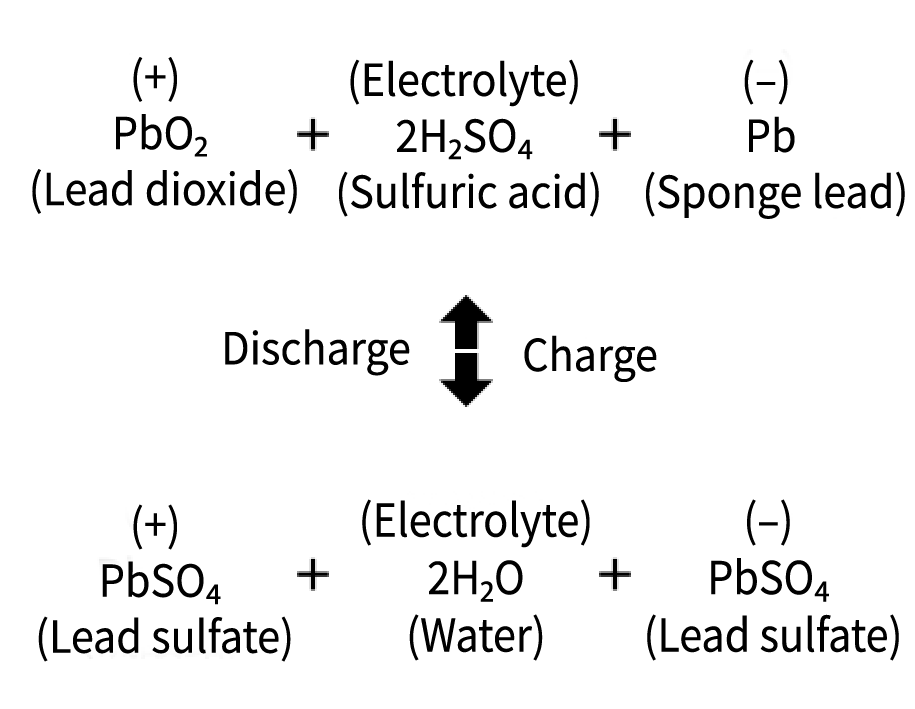
During discharge, the positive and negative plates react with sulfuric acid, reducing specific gravity.
In addition, water is generated during discharge, which further lowers electrolyte specific gravity.
Inside the battery, the following chemical reactions take place:
Even with identical batteries, lifespan varies depending on usage conditions, environment, daily maintenance, the vehicle’s charging system, and installation location.
For passenger vehicles, the recommended replacement period is approximately 3 years.
Beyond that, electrolyte loss accelerates and internal components deteriorate, so early replacement is advised.
Please consult the retailer where you purchased the battery.
Dark current refers to the discharge current that continues to flow even with the engine off and all electrical loads turned off—such as clocks and electronic memory circuits. It is typically several tens of milliamps, depending on the vehicle.
A discharge of several tens of milliamps has little effect if a short period.
However, over a long period, the battery will deeply discharge, leading to sulfation, making recovery difficult and reducing capacity and lifespan.
Example:
If a vehicle is left unused for 30 days with a dark current of 25 mA, the discharge amounts to 18 Ah, which is two-thirds of the capacity of a 40B19R battery.
Therefore, if leaving a vehicle unused for a long time, always disconnect the negative (-) terminal.
What Is Sulfation?
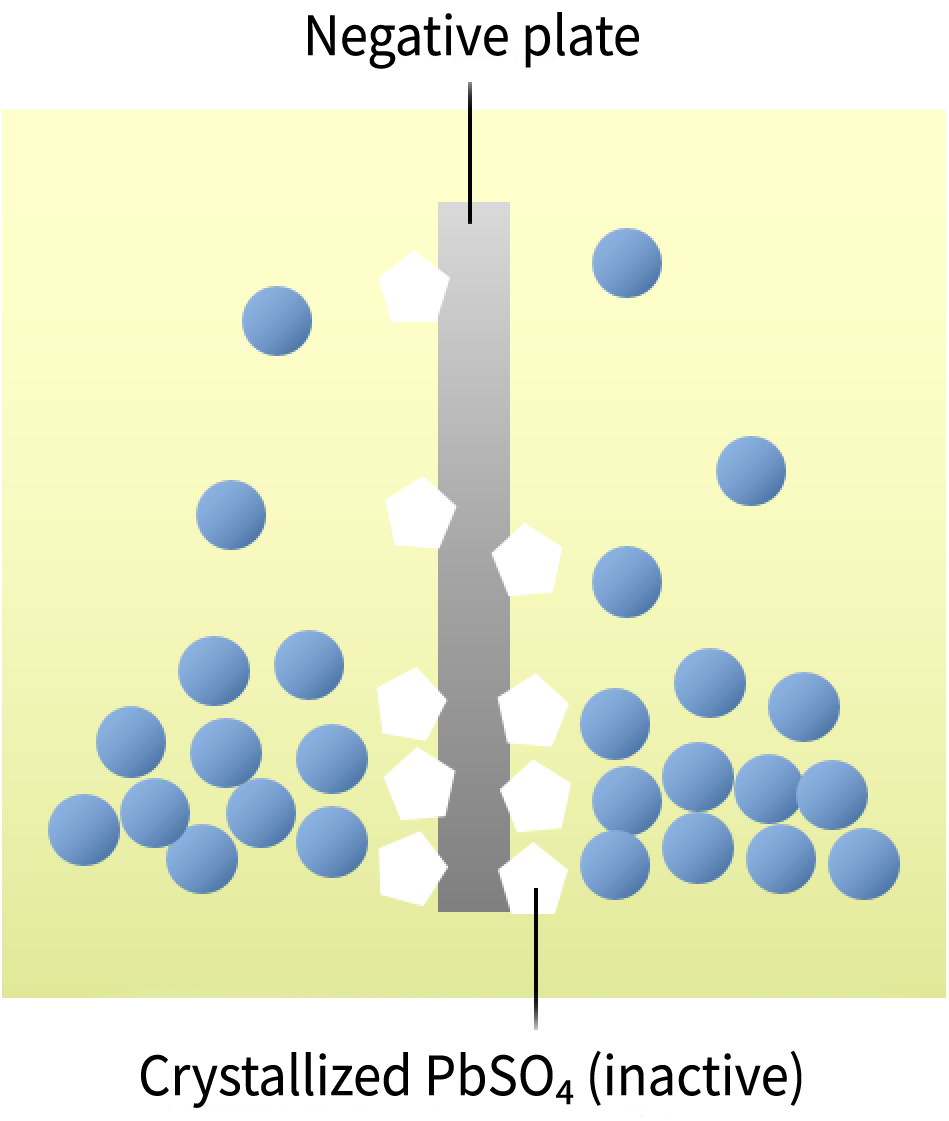
During discharge, lead sulfate (PbSO₄) is generated. Over time, it crystallizes and becomes chemically inactive. This phenomenon is called sulfation.
As sulfation progresses, battery capacity decreases, and the crystals adhere to the surface of the negative plate, reducing the contact area between the electrode plates and the electrolyte. This leads to slower charging.
A battery that remains at a low state of charge is more likely to experience accelerated sulfation.
